chlorine electron configuration|the electron configuration of potassium is 1s22s22p63s1 : Cebu Mar 23, 2023 Big-booty Busty stepmom Macey Jade gives a head to stepson before hot pussy fucking - stepmom step mom-son mom-fuck mom-pov mom-porn mom-fucks-son mom-porn mother-son mother-son-sex 5 min. 5 min Vincenzomessina -
PH0 · the electron configuration of potassium is 1s22s22p63s1
PH1 · noble gas configuration for chlorine
PH2 · ground state electron configuration for chlorine
PH3 · give the full electron configuration for sulfur
PH4 · electron configuration chart
PH5 · electron configuration calculator
PH6 · chlorine electron configuration unabbreviated
PH7 · chlorine electron configuration excited state
PH8 · Iba pa
100 - 200 Free Spins at Uptown Pokies Casino. code: COSMOS-2, COSMOS-3 The bonus is valid for depositing players. The match has no max cash out. Deposit $25 with the code COSMOS-2 and get 150% match bonus + 100 free spins on Alien Wins, Nova 7 or Cosmic Crusade.
chlorine electron configuration*******In order to write the Chlorine electron configuration we first need to know the number of electrons for the Cl atom (there are 17 electrons). When we write the configuration we'll put all 17 electrons in orbitals around the nucleus of the Chlorine atom.In order to write the Argon electron configuration we first need to know the .How to Write the Electron Configuration for Oxygen. Oxygen is the eighth element .chlorine electron configuration the electron configuration of potassium is 1s22s22p63s1Since 1s can only hold two electrons the next 2 electrons for sodium go in the 2s .
the electron configuration of potassium is 1s22s22p63s1 Mar 23, 2023 Learn how to write the complete electron configuration of chlorine (Cl) using two different methods: orbit and orbital. Compare the rules, principles, and diagram.
Learn how to write the full and abbreviated ground state electron configuration of chlorine, a nonmetal with atomic number 17. See the table of sublevels, orbitals and electrons for each energy level. Exercise \(\PageIndex{1}\): Electronic Configuration of Chlorine Atoms Using Figure \(\PageIndex{2}\) as your guide, write the electron configuration of a .
Learn the electron configuration of chlorine, a halogen with the symbol Cl and atomic number 17. Find out its properties, characteristics, history and more on this web page.
Chlorine Electron Configuration - YouTube. Wayne Breslyn. 734K subscribers. 847. 151K views 10 years ago. A step-by-step description of how to write .
Exercise \(\PageIndex{1}\): Electronic Configuration of Chlorine Atoms Using Figure \(\PageIndex{2}\) as your guide, write the electron configuration of a neutral .
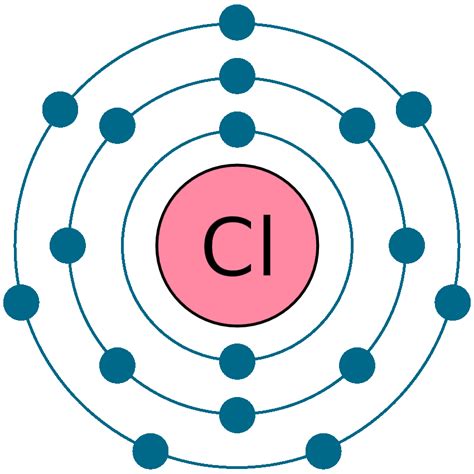
Chlorine Electron Configuration (Cl) with Orbital Diagram. For writing the Chlorine Electron Configuration you first need to check the number of electrons for the Chlorine (Cl) atom (there are . Learn how to write the electron configuration of chlorine, a non-metal in group 17 of the periodic table. Find out the number of electrons, shells, and valence electrons in chlorine and its occurrence . To write the orbital diagram for the Chlorine atom (Cl) first we need to write the electron configuration for just Cl. To do that we need to find the number.Chlorine(Cl): Chlorine is a p-block element having atomic number 17. Chlorine belongs to group 17 which is known as halogens. Electronic configuration of Group 17. The elements of group 17 have seven electrons in their outermost shell. So, the valence shell electronic configuration of group 17 is n s 2 n p 5. Electronic configuration of Chlorine:
To check the answer, verify that the subscripts add up to the atomic number. In this case, 2+2+6+2+6+2+10+6+2+1= 39 and Z=39, so the answer is correct. A slightly more complicated example is the electron . Add an electron to the anion electron configuration. For example, the ground state electronic configuration of chlorine is 1s²2s²2p⁶3s²3p⁵. For Cl −, it will be 1s²2s²2p⁶3s²3p⁶. Remove the outermost electrons in the cation, e.g. electron configuration for Mg 2+ will be 1s²2s²2p⁶.
For writing the Chlorine Electron Configuration you first need to check the number of electrons for the Chlorine (Cl) atom (there are 17 electrons in chlorine atom).When you write the configuration you will have to put all 17 electrons of the chlorine atom in the orbitals that are around the nucleus of the Chlorine atom.
The chemical symbol for the chlorine atom is Cl. The electronic configuration of chlorine is 1s 2 2s 2 2p 6 3s 2 3p 5. The distribution of electrons in the shell of the chlorine atom is 2, 8, 7. The valence electrons in chlorine are 7. A step-by-step description of how to write the electron configuration for Chlorine (Cl). In order to write the Cl electron configuration we first need to kn.
Inner transition elements are metallic elements in which the last electron added occupies an f orbital. They are shown in green in Figure 5.1.6 5.1. 6. The valence shells of the inner transition elements consist of the ( n – 2) f, the ( n – 1) d, and the ns subshells. There are two inner transition series:
mahilig ka ba sa bold? noong kabataan mo! eto na. panoorin playlist ko ng mga bold films noong dekada 80's. para manumbalik ang sarap ng . #silip #hibla .
chlorine electron configuration|the electron configuration of potassium is 1s22s22p63s1